
π-conjugated Materials for Modern Applications in
Inorganic and Bioinorganic Model Systems

Circularly Polarized Room Temperature Red Phosphorescence from a [9]-Heterohelical Terrylene Diimide
Shivangee JhaKundan Singh MehraPradip Kumar MondalCamelia DuttaJatish Kumar, Sentamizh Selven Ramalingam, Jeyaraman Sankar
J. Phys. Chem. Lett. 2025; https://doi.org/10.1021/acs.jpclett.5c03113 (Link)








Near-IR Absorbing Aza-Benzannulated Perylenebisimide-Porphyrin Tape
Ayushi Kaushik, Preetika Verma, Subhrajyoti Bhandary, Yapamanu Adithya Lakshmanna, Jeyaraman Sankar
Chem. Asian J. 2025; https://doi.org/10.1002/asia.202500881 (Link)
A Nine-Ring Fused Terrylene Diimide Exhibits Switching between Red TADF and Near-IR Room Temperature Phosphorescence
Shivangee Jha, Kundan Singh Mehra, Mandira Dey, Sujesh S, Debashree Ghosh, Pradip K Mondal, Maurizio Polentarutti, Jeyaraman Sankar
Chem. Sci. 2024 https://doi.org/10.1039/D4SC01040J (Link)
Alternatively Connected Corrole-Porphyrin Multimetallic Complexes: Synthesis, Structure and Properties
Harpal, Muthuchamy Murugavel, Jyoti Rai, Piyush Panini, R. V. Ramana Reddy, Parikshit Thakur, and Jeyaraman Sankar
Eur. J. Inorg. Chem. 2024, DOI: 10.1002/ejic.202400126 (Link)
A Bis-Porphyrin Cavitand Breathing-In to Constrict Bucky Balls
Anupam Kumari, Pradip Kumar Mondal, Preetika Verma, Paritosh Mahato, Sujesh S, Koushik Mandal, Maurizio Polentarutti, Adithya Lakshmanna Yapamanu, and Jeyaraman Sankar
Chem. Eur. J. 2024, DOI: 10.1002/chem.202401284 (Link)
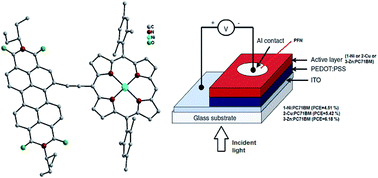
Aza-benzannulated-perylenebisimide-porphyrin dyad as an intensely absorbing donor in bulk-heterojunction organic solar cells
Ayushi Kaushik, Subhrajyoti Bhandary, Ganesh D. Sharma and Jeyaraman Sankar
Mater. Adv., 2023, 4, 6358-6366 (Link)
Copper corrole immobilized onto reduced graphene oxide: An efficient electrocatalyst for hydrogen evolution reaction
Prachi Varshney, Amit Kumar, S Sujesh, Svastik Jaiswal, and Jeyaraman Sankar
J. Porph. Phthalocyanins. 2023, 27, 702-711
The Deeper It Goes, The Brighter It Glows: NIR Emissive Nitro-Terrylene Diimides with Deep LUMOs
Kundan Singh Mehra, Shivangee Jha, Anila M Menon, Deepak Chopra and Jeyaraman Sankar
Chem. Sci. 2023, (Link)
2023 Chemical Science Hot Article Collection (Link)
Bridging the Bays, Both Ways: A Janus Butterfly-shaped Intense NIR-Emitting Terrylene Diimide
Kundan Singh Mehra, Shivangee Jha, Subhrajyoti Bhandary, Dipendranath Mandal, Ruchika Mishra and Jeyaraman Sankar
Angew. Chem. Intl. Ed. 2022, e202205600 (Link)
























Isolation and Structural Characterization of Regioisomers of Dibrominated Terrylene Diimides
Shivangee Jha, Kundan Singh Mehra, Avantika Hasija, Deepak Chopra, Ramprasad Regar and Jeyaraman Sankar
J. Org. Chem. 2022, 87, 5, 3770–3774 (Link)
Pyrene Appended Free‐base, Phosphorus(V) and Gallium(III) Corroles and Their β,β′−Linked Corrole Dimers: Synthesis, Photophysical and Electrochemical Properties
Sujesh S, Biju Basumatary, Amit Kumar and Jeyaraman Sankar
Eur. J. Inorg. Chem. 2021, 6, 540-547 (Link)
Accepting to Donate: NDI-based Small Molecule as a Donor for Bulk Heterojunction Binary Solar Cells
Ruchika Mishra, Sujesh S, Archana V. S, Ayushi Kaushik, Gaurav Gupta, Rahul Singhal, Ganesh D Sharma and Jeyaraman Sankar
Eur. J. Org. Chem. 2020, 11, 1603-1610 (Link)
Very Important Paper (VIP Article)
Electronic Modulation of Terrylene Diimides Leading to Core‐Twisting, Tunable Emission and Intermolecular Interactions
Ramprasad Regar, Kundan Singh Mehra, Rohit Bhowal and Jeyaraman Sankar
Eur. J. Org. Chem. 2019, 36, 6278-6284 (Link)
A Möbius Expanded Porphyrinoid With 2,3-Pyrrollic Connection From a Planar 𝜋-Extended BODIPY
Adiki Rajasekhar and Jeyaraman Sankar
J. Porphyrins Phthalocyanines 2019, Invited Article, Accepted
Aminophenyl-substituted Cobalt(III)Corrole: Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions
Amit Kumar, Sujesh S, Prachi Varshney, Amit Paul and Jeyaraman Sankar
Dalton Transactions 2019, 48, 11345-11351 (Link)
Tris-(manganese(III))Corrole-Porphyrin-Corrole Triad: Synthesis, Characterization and Catalytic Epoxidation
Jyoti Rai, Biju Basumatary, Subhrajyoti Bhandary, Muthuchamy Murugavel and Jeyaraman Sankar
Dalton Transactions 2019, 48, 7394-7402 (Link)
A Cell-Permeant Small Molecule for Super-Resolution Imaging of Endoplasmic Reticulum in Live Cells
Adiki Rajasekhar, Bhagaban Mallik, Vimlesh Kumar, and Jeyaraman Sankar
Org. Biomol. Chem 2019, DOI: 10.1039/C9OB00408D (Link)
Hot off the Press Article - highlighted here
A NIR Absorbing Ortho-pi-extended Perylene Bisimide as Promising Material for Bulk Heterojunction Organic Solar Cell
Ramprasad Regar, Ruchika Mishra, Rahul Singhal, Ganesh D Sharma and Jeyaraman Sankar
J. Mater. Chem. A 2019, DOI: 10.1039/C8TA10982F (Link)
Corrole-BODIPY Dyad as Small Molecule Donor for Bulk Hetero-junction Solar Cell
Ruchika Mishra, Biju Basumatary, Rahul Singhal, Ganesh D Sharma and Jeyaraman Sankar
ACS Appl. Mater. Interfaces 2018 DOI: 10.1021/acsami.8b08519 (Link)
Metal-free Annulation at Ortho- and Bay- Positions of Perylene Bisimide Leading to Lateral 𝜋-Extension with Strong NIR Absorption
Ramprasad Regar, Ruchika Mishra, Pradip Kumar Mondal and Jeyaraman Sankar
J. Org. Chem., 2018 Accepted (Link)
Sterically Hindered 5,15-Tetraphenylbenzene-Porphyrins: Syntheses, Structures, Atrop-isomerism and Photophysical Properties
R. V. Ramana Reddy, Biju Basumatary, Muthuchamy Murugavel, Karunesh Keshav, Adiki Raja Sekhar and Jeyaraman Sankar
Journal of Chemical Sciences, 2018 Accepted (MTIC Special Issue) [Invited]
Selective Imaging of Lipids in Adipocytes Using an Imidazolyl Derivative of Perylenebisimide
Ruchika Mishra, Zeeshan Mushtaq, Ramprasad Regar, Bhagaban Mallik, Vimlesh Kumar and Jeyaraman Sankar
ChemBioChem, 2018 Accepted (Link)
The Curious Case of a Parasitic Twin of the Corroles
Biju Basumatary, R. V. Ramana Reddy, Rahul, and Jeyaraman Sankar
Angew. Chem. Int. Ed. 2018, 10.1002/anie.201801555 (Link)
Bay- and ortho- ring annulated perylenediimides: Synthesis and their panchromatic absorption
Ramprasad Regar, Adiki Rajasekhar, Ruchika Mishra, and Jeyaraman Sankar
Indian J. Chem. Sec. B 2018, 57, 308-313. (Link) [Invited]
Modulation of Power Conversion Efficiency of Organic Solar Cells via Architectural Variation of a Promising Non-fullerene Acceptor
Ruchika Mishra, Ramprasad Regar, Varun Singh, Piyush Panini, Rahul Singhal, Mukhamed Lostambievich Keshtov, Ganesh D Sharma and Jeyaraman Sankar
J. Mater. Chem. A 2018, 10.1039/C7TA08533H
Evidence for a [17] pi-electronic Full-fledged Noninnocent Ga(III) Corrole Radical
Biju Basumatary, Jyoti Rai, R. V. Ramana Reddy and Jeyaraman Sankar
Chem. Eur. J, 2017, DOI: 10.1002/chem.201704457
Porphyrin Based Push-Pull Conjugates as Donors for Solution-processed Bulk Heterojunction Solar Cells: A Case of Metal-dependent Power Conversion Efficiency
Ruchika Mishra, Ramprasad Regar, Rahul Singhal, Piyush Panini, Ganesh D. Sharma and Jeyaraman Sankar
J. Mater. Chem. A., 2017, 5, 15529 - 15533
Zwitterionic BODIPYs with Larger Stokes Shift: Small Molecular Biomarkers for Live Cells
Adiki Rajasekhar, Santhosh Kumar Sariki, R. V. Ramana Reddy, Alakesh Bisai, Pushpendra K Sahu, Raghuvir S Tomar and Jeyaraman Sankar
Chem. Commun., 2017, 53, 1096-1099
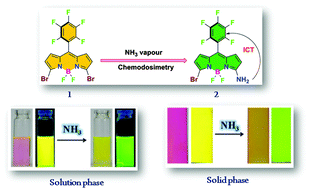
A Facile and Visual Approach for Trace Level Ammonia Vapour Detection under Ambient Conditions
Masood Ayoub Kaloo, Adiki Rajasekhar, R. V. Ramana Reddy, Ramya Sunder raman and Jeyaraman Sankar
J. Mater. Chem. C, 2016, 4, 2452-2456. (Link)
Novel structurally-tuned DAMN receptor for “in-situ” diagnosis of bicarbonate in environmental waters
Masood Ayoub Kaloo, Ramya Sunder raman and Jeyaraman Sankar
Analyst, 2016, 141, 2367-2370. (Link)
Gallium(III)Corrole-BODIPY Hybrid: Novel Photophysical Properties and First Observation of B-F---F interactions
Biju Basumataray, R. V. Ramana Reddy, Subhrajyoti Bhandary and Jeyaraman Sankar
Dalton Trans. 2015, 44, 20817-20821 (Link)
Dual-mode chemodosimetric response of dibromo-BODIPY with anions
Adiki Rajasekhar, Masood Ayoub Kaloo and Jeyaraman Sankar
Org. Biomol. Chem. 2015, 13, 10155-10161 Accepted (2015) (Link)
Reusable and Specific Proton Transfer Signalling by Inorganic Cyanide in Solution and Solid
Masood Ayoub Kaloo and Jeyaraman Sankar
Chem. Commun. 2015, 51, 14528-15531 (Link)
First Example of a Modular Porphyrinoid Assembly Capable of Stabilizing Different Metal Ions in a Single Molecular Scaffold
Muthuchamy Murugavel, R. V. Ramana Reddy, Dhananjay Dey and Jeyaraman Sankar
Chem. Eur. J. 2015, 21, 14280-14286 (Link) [Adjudged as a HOT PAPER]
Corrole-BODIPY Dyads: Synthesis, Structure, Electrochemical and Photophysical properties
Biju Basumatary, Adiki Raja Sekhar, R. V. Ramana Reddy and Jeyaraman Sankar
Inorg. Chem. 2015, 54(9), 4257-4267 (Link)
Listed as one of the top articles by Nature Index for the year 2015-16
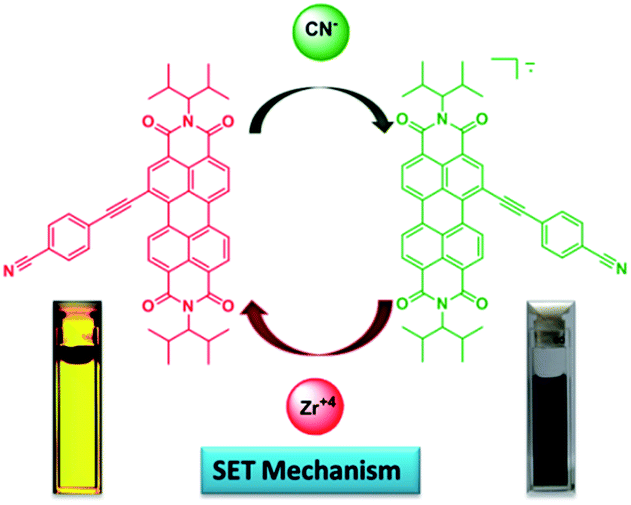
Perylenebisimide-based multi-modal cyanide recognition: molecular logic gate deciphering magnetic memory units
Masood Ayoub Kaloo, Ruchika Mishra and Jeyaraman Sankar
J. Mater. Chem. C, 2015, 3, 1640-1644 (Link)
An electron-deficient perylenebisimide has been identified as the first example of a molecular Boolean logic gate having a magnetic signalling mechanism. Cyanide ions are used as external stimuli to execute the complex logic operations such as IMP (implication function) and INH (inhibition function) and it has been demonstrated that the performed operations can be monitored via both optical and electron paramagnetic signalling pathways. Particularly, the signalling mechanism is completely reversible and non-destructive. In comparison to the optical signalling pathway, the current sensing mechanism is shown to be highly efficient and sensitive.
Novel azepino-perylenebisimides: synthesis, structure, and properties
Ruchika Mishra, Piyush Panini and Jeyaraman Sankar
Org. Lett., 2014, 16, 3994-3997 (Link)
The first example of an azepine ring formation by counterintuitive nucleophilic participation of DBU was observed at the sterically crowded bay area of electron-deficient perylenebisimide (PBI). This is also a rare example of the formation of a seven-membered ring via two consecutive C-N bond formations in a single step. Azepino-PBIs reveal panchromatic absorption covering the whole visible region. Further novelty of these PBIs lies within the fact that their photophysical characteristics can easily be modulated by suitable substituents.
Highlighted in DOI: 10.1021/acs.chemrev.6b00181

Aliphatic Amine Discrimination by Pentafluorophenyl Dibromo BODIPY
Adiki Rajasekhar, Masood Ayoub Kaloo and Jeyaraman Sankar
Chem. Asian J., 2014, 9, 2422-2426 (Link)
Two new fluorescent BODIPY dyes have been designed and synthesized. They dyes differ in theirmeso substituents, which have different electronic properties. Their selective reactivity towards an Ar-SN2 reaction has been explored as a potential basis for colorimetric and fluorescent discrimination of primary, secondary and tertiary aliphatic amines. This dual-mode, instantaneous recognition event is unprecedented.

A selective fluoride sensor and a digital processor with “Write–Read–Erase–Read” behaviour
Basant Kumar, Masood Ayoub Kaloo, Adiki Rajasekhar and Jeyaraman Sankar
Dalton Trans., 2014, 43, 16164-16168 (Link)
An easy-to-synthesize Schiff base as a selective and colorimetric fluoride sensor via modulation of intramolecular charge transfer (ICT) has been demonstrated. A typical dual-ion binding property along with its distinct reversibility has been explored for YES, NOR and INH logic functions and a potential “Write–Read–Erase–Read” mimic..

Tuning the Electronic Nature of Mono-Bay Alkynyl–Phenyl-Substituted Perylene Bisimides: Synthesis, Structure, and Photophysical Properties
Ruchika Mishra, Jong Min Lim, Minjung Son, Piyush Panini, Dongho Kim and Jeyaraman Sankar
Chem. Eur. J., 2014, 20, 5776-5786 (Link)
Perylene bisimide (PBI) derivatives with various alkynyl–phenyl substituents at a single bay position have been synthesised by Sonogashira coupling. NMR spectroscopic studies reveal the unsymmetric nature of the dyads. All of the dyads undergo two reversible reductions, which demonstrates their structural and electrochemical rigidity. The synthesised dyads show a remarkable redshift in their absorption maxima and sharp vibronic progression. Electron-rich substituents facilitate efficient charge transfer from the substituent HOMO to the electron-deficient PBI core. Despite several previous reports on the structural characterisation of 1,7-disubstituted PBI derivatives, we present the first structural characterisation of a mono-bay ethynyl-phenyl substituted PBI. The solid-state structure of the phenyl derivative has a flat PBI core without any noticeable steric constraints from the substituents, as predicted. In contrast, single-crystal X-ray analysis for the mono-bromo bay-substituted PBI shows that the bromine substituent is not in the plane of the PBI core.
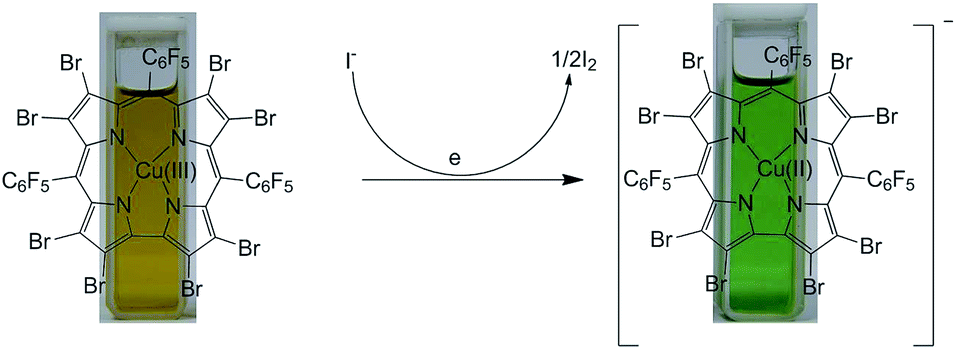
Selective iodide chemosensing through a redox-active Cu-corrole
Biju Basumatary, Masood Ayoub Kaloo, Vineet Kumar Singh, Ruchika Mishra, Muthuchamy Murugavel and Jeyaraman Sankar
RSC Adv., 2014, 4, 28417-28420 (Link)
An electron deficient Cu(III)-corrole (B) has been identified as a highly selective colorimetric sensor for iodide. A one-electron redox couple between iodide and Cu(III)-corrole serves as the basis for selectivity. The proposed mechanism has been supported by UV-vis and 19F NMR studies and further by unambiguous identification of Cu(II) species through EPR.
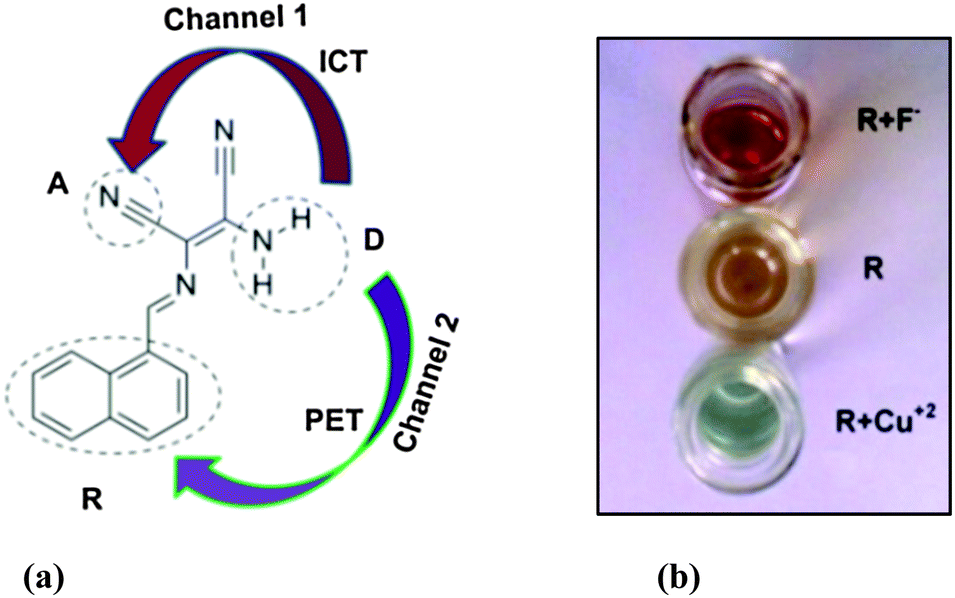
A molecular Boolean mimic with OR, NOR, YES and INH functions: dual-ion recognition driven fluorescence “turn on”
Masood Ayoub Kaloo and Jeyaraman Sankar
New J. Chem., 2014, 38, 923-926 (Link)
Naphthyl-based Schiff base (R) has been explored as a dual-ion receptor for Cu+2 and F− with a fluorescence “turn on” response at a single wavelength. The changes are attributed to the modulation of ICT and PET processes. The recognition behaviour demonstrates R as a Boolean mimic for computation of OR, NOR, YES and INH functions with a convenient reset mechanism.
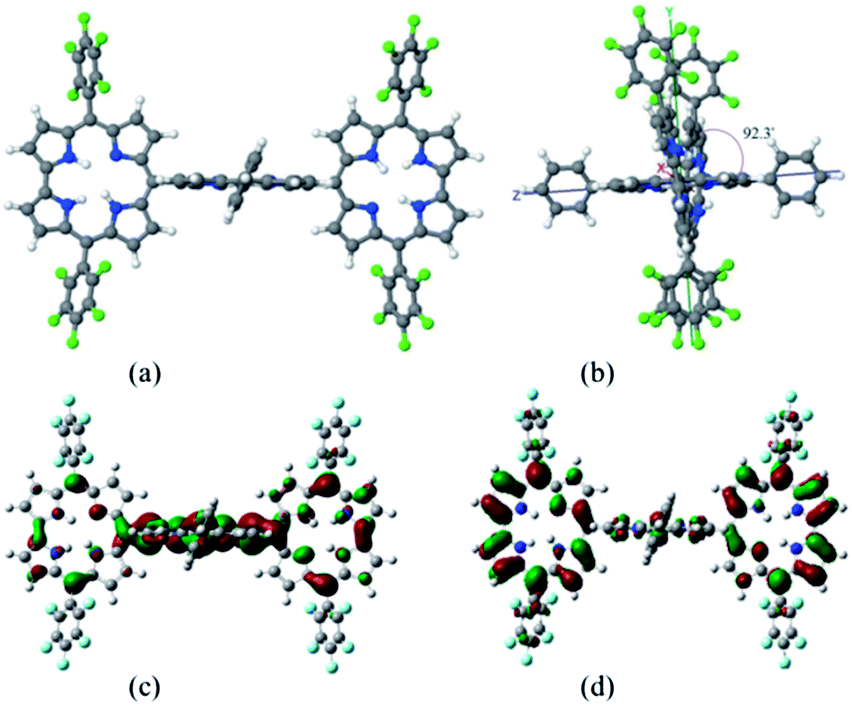
A new meso–meso directly-linked corrole–porphyrin–corrole hybrid: synthesis and photophysical properties
Muthuchamy Murugavel, R. V. Ramana Reddy and Jeyaraman Sankar
RSC Adv., 2014, 4, 13669-13672 (Link)
A first example of a directly-linked corrole–porphyrin–corrole (Cor–Por–Cor) triad has been targeted and synthesized. The new hybrid has been fully characterized by 1H NMR, 19F NMR and 2D NMR spectroscopy. The fluorescence quantum yield of the triad is three times higher than that ofmeso–meso directly linked porphyrin trimers. All the three chromophores assume a near orthogonal geometry with respect to the neighbour.
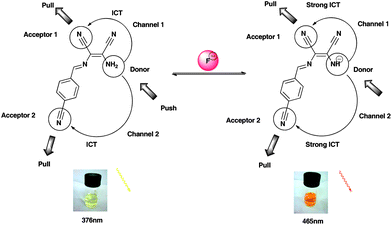
Exclusive fluoride ion recognition and fluorescence “turn-on” response with a label-free DMN Schiff base
Masood Ayoub Kaloo and Jeyaraman Sankar
Analyst 2013, 138, 4760-4763 (Link) [One of the most accessed papers]
A label-free DMN Schiff base has been explored as a highly selective and sensitive fluoride ion receptor. Fluoride-induced deprotonation results in a charge transfer (CT) transition red shifted with a fluorescence ‘turn-on’. Anion selectivity can be tuned by the electron push–pull property of substituents at the phenyl para-position. Selectivity for F− is attributed to the suitable acidity of –NH2.
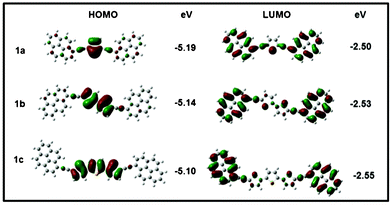
Thienyl-linked pyrenes: new green-emitting triads
Ruchika Mishra and Jeyaraman Sankar
RSC Adv., 2014, 43, 10658-10661 (Link)
Novel bis-pyrenyl systems connected through oligothienyl groups have been synthesized, characterized and their optical and electrochemical properties have been studied. By increasing the conjugation length of the spacers, the emission from these systems can be tuned to longer wavelengths. Apart from this, the synthesized molecules have interesting photophysical properties owing to the possible intramolecular charge transfer.
Publications (prior to joining IISERB)
15. Temperature Dependent Molecular Locking of meso- Hexakis (pentafluorophenyl) [28]Hexaphyrins (1.1.1.1.1.1) into Möbius Structures
Kim, K. S.; Yoon, Z. S.; Ricks, A.; Shin, Jae-Yoon, Mori, S.; Sankar, J.; Saito, S.; Jung, Y. M.; Wasielewski, M.; Osuka, A.; Kim, D.
J. Phys. Chem. 2009, 113 (16), 4498.
14. Unambiguous Identification of Möbius Aromaticity for meso-Aryl Substituted [28]Hexaphyrins (1.1.1.1.1.1)
Sankar, J.; Mori, S.; Saito, S.; Rath, H.; Suzuki, M.; Inokuma, Y.; Shinokubo, H.; Kim, K. S.; Yoon, Z. S.; Shin, J-Y.; Lim, J. M.; Matsuzaki, Y.; Osamu, M.; Muranaka, A.; Kobayashi, N.; Kim, D.; Osuka, A.
J. Am. Chem. Soc. 2008, 130, 13568.
13. 2, 5-Thienylene-Bridged Triangular and Linear Porphyrin Trimers
Song, J.; Jang, S. Y.; Yamaguchi, S.; Sankar, J.; Hiroto, S.; Aratani, N.; Shin, J-Y.; Easwaramoorthi, S.; Kim, K. S.; Kim, D.; Shinokubo, H.; Osuka, A.
Angew. Chem.. Int. Ed. 2008, 47, 6004.
12. meso-meso Linked Corrole Dimers with Modified Cores: Synthesis, Characterization and Properties
Sankar, J.; Rath, H.; PrabhuRaja, V.; Gokulnath, S.; Chandrashekar, T. K.; Purohit, C. S.; Verma, S.
Chem. Eur. J. 2007, 13, 105.
11. Smaragdyrin-Azobenzene Conjugates: Syntheses, Structure, and Spectral and Electrochemical Properties
Gokulnath, S; PrabhuRaja, V; Sankar, J; Chandrashekar, T. K.
Eur. J. Org. Chem. 2007, 1, 191.
10. Core-Modified Expanded Porphyrins with Large Third-Order Nonlinear Optical Response
Rath, H.; Sankar, J.; PrabhuRaja, V.; Chandrashekar, T. K.; Nag, A.; Goswami, D.
J. Am. Chem. Soc. 2005, 127, 11608.
9. Figure-eight Aromatic Core-modified Octaphyrins with six meso Links: Synthesis and Structural Characterization
Rath, H.; Sankar, J.; PrabhuRaja, V.; Chandrashekar, T. K.; Joshi, B.; Roy, R.
Chem. Comm. 2005, 3343.
8. Aromatic Core-Modified Twisted Heptaphyrins [1.1.1.1.1.1.0]: Syntheses and Structural Characterization
Rath, H.; Sankar, J.; PrabhuRaja, V.; Chandrashekar, T. K.; Joshi, B. S.
Org. lett. 2005, 24, 5445.
7. Syntheses of Core-Modified Corroles by Three Different [3+1] Methodologies
Sankar, J.; Rath, H.; PrabhuRaja, V.; Chandrashekar, T.K.; Vittal, J. J.
J. Org. Chem. 2004, 69, 5135.
6. Oxasmaragdyrin-Ferrocene and Oxacorrole-Ferrocene Conjugates: Synthesis, Structure, and Nonlinear Optical Properties
Venkatraman, S.; Kumar, R.; Sankar, J.; Chandrashekar, T. K.; Sendhil, K.; Vijayan, C.; Kelling, A.; Senge, M. O.
Chem. Eur. J. 2004, 10, 1423.
5. Core-Modified Hexaphyrins; Characterization of Two- and Four-Ring Inverted 26p Aromatic Macrocycles
Rath, H.; Anand, V. G.; Sankar, J.; Venkatraman, S.; Chandrashekar, T. K.; Joshi, B.; Khetrapal, C. L.; Schilde, U.; Senge, M. O.
Org. Lett. 2003, 5, 3531.
4. First Structural Characterization of Core-Modified 10,15-meso Aryl Azuliporphyrins: Observation of C-H---p Interaction Between Pyrrole b-CH and Mesityl Ring
Venkatraman, S.; Anand, V. G.; PrabhuRaja, V.; Rath, H.; Sankar, J. Chandrashekar, T.K.; Teng, W.; Senge, K. R.
Chem. Comm. 2002, 16, 1660.
3. Core-Modified Oxybenziporphyrins: New Aromatic Ligands for Metal-Carbon Bond Activation
Venkatraman, S.; Anand, V. G.; Pushpan, S. K.; Sankar, J.; Chandrashekar, T.K.
Chem. Comm. 2002, 5, 462.
2. Porphyrins in Photodynamic Therapy – A Search for Ideal Photosensitizers
Pushpan, S. K.; Venkatraman, S.; Anand, V. G.; Sankar, J.; Parameswaran, D.; Ganesan, S.; Chandrashekar, T. K.
Current Medicinal Chemistry-Anti Cancer Agents. 2002, 2, 187. (Review).
1. Modified Corroles With One Meso-Free Carbon: Synthesis and Characterization
Sankar, J.; Anand, V. G.; Venkatraman, S.; Rath, H.; Chandrashekar, T. K.
Org. Lett. 2002, 4, 4233.


